Efzofitimod is a first-in-class biologic immunomodulator in clinical development for the treatment of interstitial lung disease (ILD), a group of immune-mediated disorders that can cause inflammation and progressive fibrosis, or scarring, of the lungs. We are currently investigating efzofitimod in the Phase 3 EFZO-FIT™ study in patients with pulmonary sarcoidosis, a major form of ILD, and the Phase 2 EFZO-CONNECT™ study in patients with systemic sclerosis (SSc, or scleroderma)-related ILD.
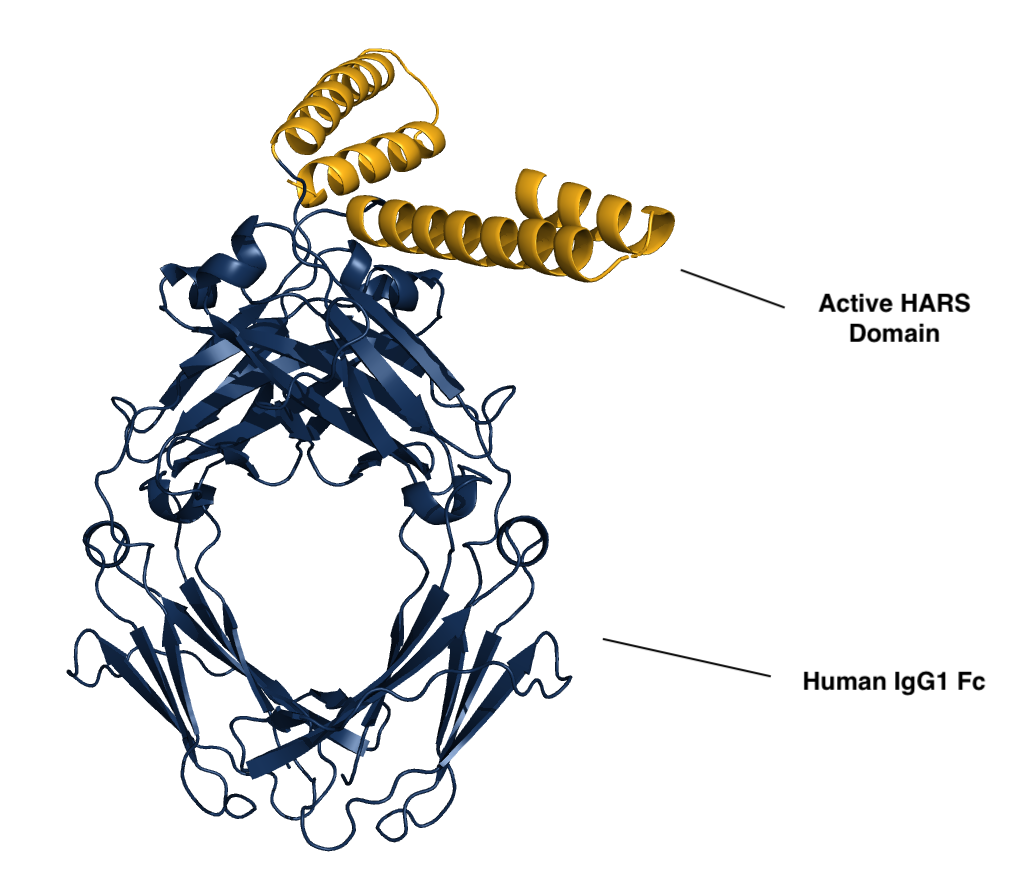
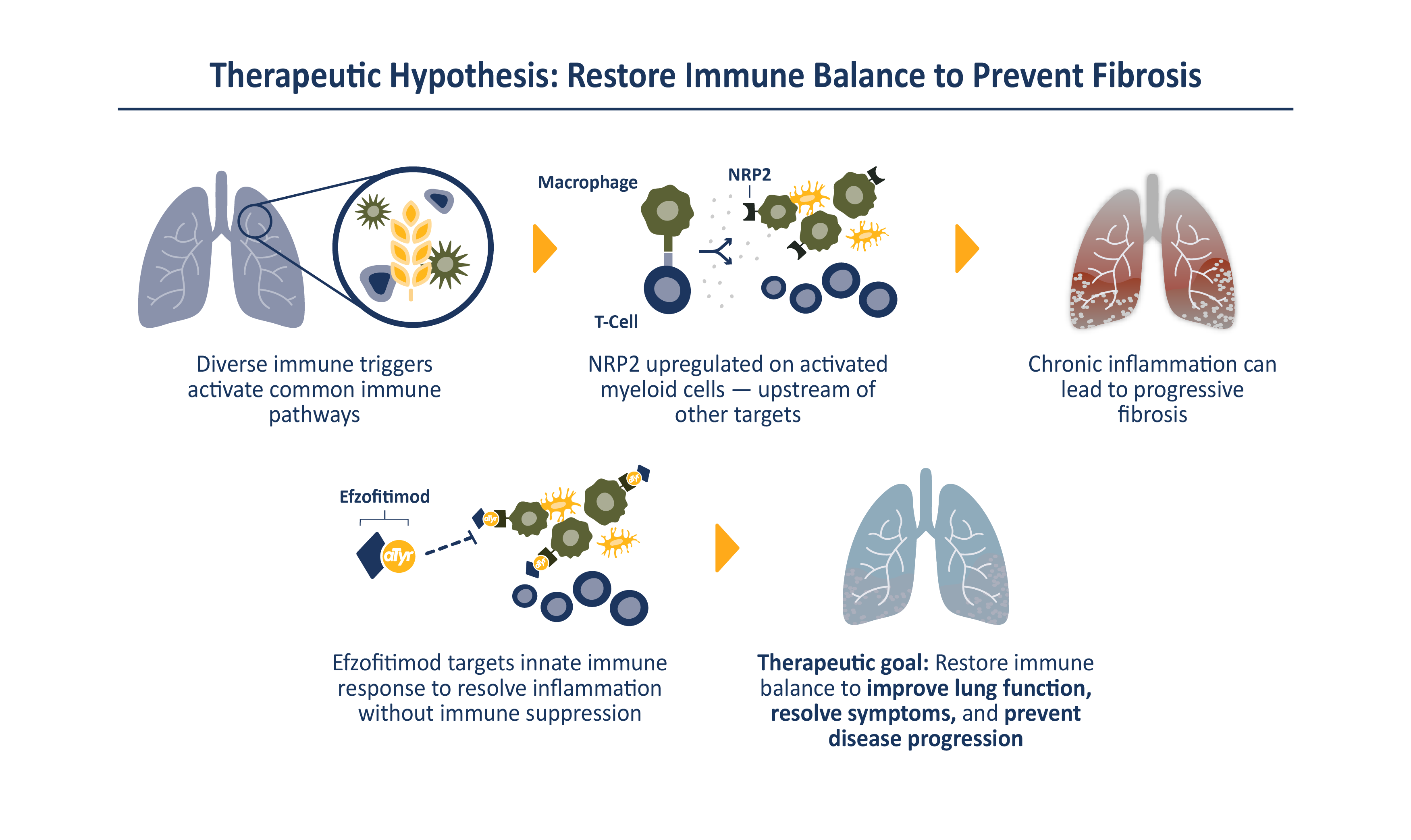
Efzofitimod is a tRNA synthetase-derived therapy based on a naturally occurring, lung enriched, splice variant of histidyl-tRNA synthetase (HARS). Efzofitimod selectively modulates activated myeloid cells through neuropilin-2 (NRP2) to resolve inflammation without immune suppression and potentially prevent the progression of fibrosis. NRP2 is a new immune target in ILD that is upstream of other therapeutic targets, which allows it to hit multiple inflammatory and pro-fibrotic pathways, addressing the complex immune pathology observed in ILD.
Efzofitimod directly targets the immune pathology common to many forms of ILD. NRP2 is upregulated on activated myeloid cells that drive inflammation and is enriched in the granulomas of sarcoidosis patients and skin lesions from patients with SSc-ILD. Efzofitimod promotes anti-inflammatory macrophage activity to downregulate multiple-pro-inflammatory cytokines, including TNFɑ, IL-6 and MCP-1, and receptors such as CD-14, that are dysregulated in ILD. In multiple animal models of ILD, efzofitimod reduced inflammation and fibrosis and was shown to prevent sarcoid granuloma formation in vitro. Clinical proof-of-concept was established for efzofitimod in a Phase 1b/2a study in patients with pulmonary sarcoidosis.
By selectively targeting activated myeloid cells to dampen multiple pro-inflammatory pathways, efzofitimod has the potential to provide greater efficacy with an improved safety profile compared to the current standard of care for ILD.
Target Populations
Interstitial Lung Disease
ILD are a group of immune-mediated disorders which can cause progressive fibrosis, or scarring, of the lung. Chronic inflammation and tissue scarring results in stiffness in the lungs which makes it difficult to breathe and get oxygen to the bloodstream. Lung damage from ILD can worsen over time and is often irreversible. There are over 200 different types of ILD, with four main types comprising roughly 80% of all patients: pulmonary sarcoidosis, chronic hypersensitivity pneumonitis (CHP), connective tissue disease-associated ILD (CTD-ILD) and idiopathic pulmonary fibrosis (IPF). Despite diverse disease triggers, ILD share common characteristics including chronic inflammation which can lead to progressive fibrosis. All ILD are associated with debilitating symptoms and poor outcomes, with some patients having worse life expectancy than common forms of cancer. Current treatment options for ILD are limited and generally focus on controlling inflammation, with anti-fibrotic therapies approved for use in select patients. No disease modifying treatments are currently available. We estimate there are over 500,000 ILD patients in the United States and upwards of 3 million globally.
PULMONARY SARCOIDOSIS
Sarcoidosis is an inflammatory disease of unknown cause, characterized by the formation of granulomas, clumps of inflammatory cells, in one or more organs in the body. Sarcoidosis affects people of all ages, with incidence peaking between 20 and 39 years of age. The disorder usually begins in the lungs, skin or lymph nodes, but can affect almost any organ. Sarcoidosis in the lungs is called pulmonary sarcoidosis and affects over 90% of patients. Approximately 200,000 Americans and 1.2 million people globally currently live with pulmonary sarcoidosis. The prognosis for patients with pulmonary sarcoidosis ranges from benign and self-limiting to chronic, debilitating disease and death. Current standard of care for patients with sarcoidosis involves treatment with corticosteroids and other immunosuppressive therapies, with limited evidence of efficacy and serious long-term toxicity. There remains a need for novel treatment options for sarcoidosis patients with progressive disease.
SSC-ILD
Systemic sclerosis (SSc, or scleroderma, is a chronic, progressive, autoimmune disease characterized by inflammation and fibrosis of connective tissues throughout the body, including the skin and other internal organs. SSc that occurs in the lungs is called SSc-ILD and is a major form of CTD-ILD. It is estimated that approximately 100,000 people in the United States are affected by SSc and up to 80% may develop ILD. SSc-ILD is caused by chronic inflammation in the lungs and, if left untreated, can result in scarring, or fibrosis, that causes permanent loss of lung function. ILD is the primary cause of death in patients with SSc. Current treatment options are limited. They mainly focus on slowing lung function decline, do not improve patient symptoms and are associated with significant toxicity. New treatments are needed that can stabilize or improve lung function and patient quality of life.